Хэрэглэгч:Bilguun.alt/Ноорог/Полимеразын гинжин урвал

Полимеразын гинжин урвал (ПГУ) нь молекул биологид өргөн хэрэглэгддэг шинжилгээний арга юм. Үүний нэр нь ДНХ полимераз гэдэгээс гаралтай. ДНХ полимеразаар ДНХ-ын өчүүхэн хэсгийг хиймэл орчинд буюу (инвитрод) ферментийн репликац ашиглан олшруулдаг (ампликац). Полимеразын гинжин урвал явагдахад өөрөө өөрийгөө үүсгэсэн ДНХ нь олшруулалтанд хэрэглэгдэх загвар болдог. Улмаар ДНХ-ын загвар экспоненциалаар олшрох гинжин урвал хөдөлгөөнд орж эхэлдэг. ПГУ-ын тусламжтай ДНХ-ийн өчүүхэн хэсгийн ганц эсвэл цөөн хуулбарыг олшруулж хэдэн сая болон түүнээс дээш ДНХ-ийн хэсгийг гарган авах боломжтой. ПГУ-ыг ДНХ-ын хэлбэрийг өөрчлөхгүйгээр гүйцэтгэх мөн боломжтой бөгөөд үүнийг өргөжүүлэн хөгжүүлж генетикийн инженерчлэлийн өргөн талбарт хэрэглэж болдог.
Бараг бүх ПГУ-ын арга нь дулаанд тогтвортой ДНХ полимеразыг ашигладаг. Тухайлбал Термус акуатикус бактериас гаргаж авсан фермент болох Таку полимеразыг ашигладаг. Энэхүү ДНХ-ийн полимераз нь дан хэлхээт ДНХ-г загвар болгон ашиглаж ДНХ-г бүрэлдүүлэгч нэгж болох нуклеотидуудаас шинэ ДНХ-ийн хэлхээг ферментлэх замаар бүрэлдүүлдэг бөгөөд үүнд ДНХ-ийн олигонуклеотидуудыг (мөн ДНХ праймерууд ч гэж нэрлэдэг) ДНХ-ийн нийлэгжилтийг эхлүүлхэд хэрэглэдэг. ПГУ-ын аргуудын ихэнх нь термал циклерийг ашигладаг бөгөөд энэ төхөөрөмж нь ПГУ-ын дээжийг тодорхой температурын шатлалуудын дагуу явуулах үүднээс ээлжлэн халааж, хөргөх процессыг явуулдаг. Эдгээр өөр өөр температурын шатлалууд нь ДНХ-ийн хос спиралын хэлхээг задлахад (ДНХ хайлалт) шаардлагатай бөгөөд ДНХ полимеразыг ашиглан ДНХ-ийн нийлэгжилтийг эхлүүлж бай ДНХ-г сонгон олшруулах боломжыг олгодог. ПГУ-ын хүчин чадал болон сонголт нь гол төлөв праймеруудыг хэрхэн сонгохоос хамаардаг ба праймерууд нь олшруулахаар сонгосон ДНХ-ийн муж болон ашиглах термо циклийн горимоосоо маш ихээр хамаардаг.
Developed in 1983 by Kary Mullis,[1] PCR is now a common and often indispensable technique used in medical and biological research labs for a variety of applications. These include DNA cloning for sequencing, DNA-based phylogeny, or functional analysis of genes; the diagnosis of hereditary diseases; the identification of genetic fingerprints (used in forensics and paternity testing); and the detection and diagnosis of infectious diseases. Mullis won the Nobel Prize for his work on PCR.[2]
ПГУ-ын зарчим болон ажиллах горим
[засварлах | кодоор засварлах]

Полимеразын гинжин урвалыг ДНХ-ийн хэлхээний тодорхой хэсгийг (бай ДНХ) олшруулахад ашигладаг. Тэр тодорхой хэсэг нь дан ганц гени, генийн хэсэг, эсвэл кодлогдоогүй дараалал байж болно. Ихэнх ПГУ-ын аргуудын нийтлэг тал нь дээд тал нь 10 кило хос суурь (кс) хүртэл урттай хэрчимүүдийг олшруулдаг. Гэвч зарим аргууд нь 40 кс хэмжээтэй хэрчимүүдийг олшруулах боломжтой байдаг.[3]
Үндсэн ПГУ-ыг тавихад хэд хэдэн найрлага болон урвалж шаардлагатай болдог.[4] Эдгээр найрлагуудыг дурдвал:
- Олшруулахаар төлөвлөсөн ДНХ-ийн хэсгийг агуулсан ДНХ-ийн загвар.
- ДНХ-ийн мужын 5' (таван праймын) болон 3' (гурван праймын) төгсгөлүүд дээрх ДНХ-ын хэсгийг нөхдөг нэг болон эсвэл хэд хэдэн праймерууд.
- ДНХ полимераз тухайлбал Таку полимераз эсвэл 70°C орчимд тохиромжтой өөр ДНХ-ийн полимераз.
- Дезоксирибонуклеотид трифосфат (дНТФ) нь тулгуур өрөгч буюу эдгээрээс ДНХ-ийн полимеразаар шинэ ДНХ хэлхээг нийлэгжүүлдэг.
- Buffer solution, providing a suitable chemical environment for optimum activity and stability of the DNA polymerase.
- Divalent cations, magnesium or manganese ions; generally Mg2+ is used, but Mn2+ can be utilized for PCR-mediated DNA mutagenesis, as higher Mn2+ concentration increases the error rate during DNA synthesis[5]
- Monovalent cation potassium ions.
The PCR is commonly carried out in a reaction volume of 15-100 μl in small reaction tubes (0.2-0.5 ml volumes) in a thermal cycler. The thermal cycler allows heating and cooling of the reaction tubes to control the temperature required at each reaction step (see below). Thin-walled reaction tubes permit favorable thermal conductivity to allow for rapid thermal equilibration. Most thermal cyclers have heated lids to prevent condensation at the top of the reaction tube. Older thermocyclers lacking a heated lid require a layer of oil on top of the reaction mixture or a ball of wax inside the tube.
Ажиллах зарчим
[засварлах | кодоор засварлах]
ПГУ нь гол төлөв 20-оос 35 хүртэл давтагдах температурын өөрчлөлтүүдийн цувралаас бүрдэнэ. Эдгээр өөрчлөлтүүдийг мөчлөг буюу цикл гэдэг. Мөчлөг бүр нь 2-3 удаагийн тасралттай (дискрет) температуруудын шатлалаас бүрддэг. Хамгийн түгээмэл ПГУ нь температурын гурван шатлалтай мөчлөгүүдээр явагддаг (Зураг 2). Мөчлөг бүр нь ихэвчлэн өндөрт хэмд (>90°C) температурын нэг шатлалаар эхлэх бөгөөд үүнийг барилт гэдэг. Улмаар эцсийн бүтээгдхүүний өргөтгөл эсвэл түр хадгалалтын төгсгөлд дахин нэг барилт явагддаг. Мөчлөг бүрд ашиглагдах температурын хэмжээ болон тухайн температурт барих хугацаа зэрэг нь олон хүчин зүйлсээс хамаардаг. Эдгээрт ДНХ-ийн нийлэгжилтэнд хэрэглэгдэх фермент, урвал дахь дНТФ (дезоксирибонуклеотид трифосфат) болон хоёр валентын ионуудын найрлага, мөн праймеруудын хайлах температур (Tm) зэрэг нь ордог.[6]
- Эхлэх шатлал: Энэ шатлалд урвалыг 94-96°C (хэрэв дулаанд маш тогтвортой полимераз хэрэглэгдэж буй үед 98°C хүртэл) температурт хүртэл халаана. Энэ температурт 1-9 минут орчим барьдаг. Энэ нь зөвхөн халуун эхлүүлэлттэй ПГУ-аар (ХЭ ПГУ) тавигддаг дулааны идэвхжүүлэлт шаарддаг ДНХ полимеразуудад ашиглагддаг.[7]
- Denaturation step: This step is the first regular cycling event and consists of heating the reaction to 94-98°C for 20-30 seconds. It causes melting of DNA template and primers by disrupting the hydrogen bonds between complementary bases of the DNA strands, yielding single strands of DNA.
- Annealing step: The reaction temperature is lowered to 50-65°C for 20-40 seconds allowing annealing of the primers to the single-stranded DNA template. Typically the annealing temperature is about 3-5 degrees Celsius below the Tm of the primers used. Stable DNA-DNA hydrogen bonds are only formed when the primer sequence very closely matches the template sequence. The polymerase binds to the primer-template hybrid and begins DNA synthesis.
- Extension/elongation step: The temperature at this step depends on the DNA polymerase used; Taq polymerase has its optimum activity temperature at 75-80°C,[8][9] and commonly a temperature of 72°C is used with this enzyme. At this step the DNA polymerase synthesizes a new DNA strand complementary to the DNA template strand by adding dNTP's that are complementary to the template in 5' to 3' direction, condensing the 5'-phosphate group of the dNTPs with the 3'-hydroxyl group at the end of the nascent (extending) DNA strand. The extension time depends both on the DNA polymerase used and on the length of the DNA fragment to be amplified. As a rule-of-thumb, at its optimum temperature, the DNA polymerase will polymerize a thousand bases in one minute.
- Final elongation: This single step is occasionally performed at a temperature of 70-74°C for 5-15 minutes after the last PCR cycle to ensure that any remaining single-stranded DNA is fully extended.
- Final hold: This step at 4-15°C for an indefinite time may be employed for short-term storage of the reaction.
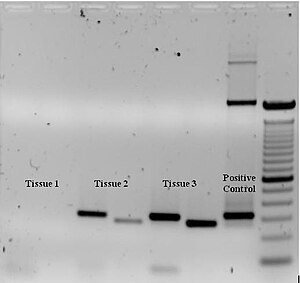
To check whether the PCR generated the anticipated DNA fragment (also sometimes referred to as the amplimer or amplicon), agarose gel electrophoresis is employed for size separation of the PCR products. The size(s) of PCR products is determined by comparison with a DNA ladder (a molecular weight marker), which contains DNA fragments of known size, run on the gel alongside the PCR products (see Fig. 3).
PCR optimization
[засварлах | кодоор засварлах]
In practice, PCR can fail for various reasons, in part due to its sensitivity to contamination causing amplification of spurious DNA products. Because of this, a number of techniques and procedures have been developed for optimizing PCR conditions.[10][11] Contamination with extraneous DNA is addressed with lab protocols and procedures that separate pre-PCR reactions from potential DNA contaminants.[4] This usually involves spatial separation of PCR-setup areas from areas for analysis or purification of PCR products, and thoroughly cleaning the work surface between reaction setups. Primer-design techniques are important in improving PCR product yield and in avoiding the formation of spurious products, and the usage of alternate buffer components or polymerase enzymes can help with amplification of long or otherwise problematic regions of DNA.
Application of PCR
[засварлах | кодоор засварлах]Isolation of genomic DNA
[засварлах | кодоор засварлах]PCR allows isolation of DNA fragments from genomic DNA by selective amplification of a specific region of DNA. This use of PCR augments many methods, such as Southern and northern blotting and DNA cloning, that require large amounts of DNA, representing a specific DNA region. PCR supplies these techniques with high amounts of pure DNA, enabling analysis of DNA samples even from very small amounts of starting material.
Other applications of PCR include DNA sequencing to determine unknown PCR-amplified sequences in which one of the amplification primers may be used in Sanger sequencing, isolation of a DNA sequence to expedite recombinant DNA technologies involving the insertion of a DNA sequence into a plasmid or the genetic material of another organism. PCR may also be used for genetic fingerprinting; a forensic technique used to identify a person or organism by comparing experimental DNAs through different PCR-based methods.
Some PCR 'fingerprints' methods have high discriminative power and can be used to identify genetic relationships between individuals, such as parent-child or between siblings, and are used in paternity testing (Fig. 4). This technique may also be used to determine evolutionary relationships among organisms.

Amplification and quantitation of DNA
[засварлах | кодоор засварлах]Because PCR amplifies the regions of DNA that it targets, PCR can be used to analyze extremely small amounts of sample. This is often critical for forensic analysis, when only a trace amount of DNA is available as evidence. PCR may also be used in the analysis of ancient DNA that is thousands of years old. These PCR-based techniques have been successfully used on animals, such as a forty-thousand-year-old mammoth, and also on human DNA, in applications ranging from the analysis of Egyptian mummies to the identification of a Russian Tsar[12].
Viral DNA can likewise be detected by PCR. The primers used need to be specific to the targeted sequences in the DNA of a virus, and the PCR can be used for diagnostic analyses or DNA sequencing of the viral genome. The high sensitivity of PCR permits virus detection soon after infection and even before the onset of disease. Such early detection may give physicians a significant lead in treatment. The amount of virus ("viral load") in a patient can also be quantified by PCR-based DNA quantitation techniques (see below).
Quantitative PCR methods allow the estimation of the amount of a given sequence present in a sample – a technique often applied to quantitatively determine levels of gene expression. Real-time PCR is an established tool for DNA quantification that measures the accumulation of DNA product after each round of PCR amplification.
Үндсэн PCR аргачлалын хувилбарууд
[засварлах | кодоор засварлах]- Allele-өвөрмөц PCR: This diagnostic or cloning technique is used to identify or utilize single-nucleotide polymorphisms (SNPs) (single base differences in DNA). It requires prior knowledge of a DNA sequence, including differences between alleles, and uses primers whose 3' ends encompass the SNP. PCR amplification under stringent conditions is much less efficient in the presence of a mismatch between template and primer, so successful amplification with a SNP-specific primer signals presence of the specific SNP in a sequence.[13] See SNP genotyping for more information.
- Assembly PCR: Assembly PCR is the artificial synthesis of long DNA sequences by performing PCR on a pool of long oligonucleotides with short overlapping segments. The oligonucleotides alternate between sense and antisense directions, and the overlapping segments determine the order of the PCR fragments thereby selectively producing the final long DNA product.[14]
- Asymmetric PCR: Asymmetric PCR is used to preferentially amplify one strand of the original DNA more than the other. It finds use in some types of sequencing and hybridization probing where having only one of the two complementary stands is required. PCR is carried out as usual, but with a great excess of the primers for the chosen strand. Due to the slow (arithmetic) amplification later in the reaction after the limiting primer has been used up, extra cycles of PCR are required.[15] A recent modification on this process, known as Linear-After-The-Exponential-PCR (LATE-PCR), uses a limiting primer with a higher melting temperature (Tm) than the excess primer to maintain reaction efficiency as the limiting primer concentration decreases mid-reaction.[16]
- Colony PCR: Bacterial colonies (E.coli) can be rapidly screened by PCR for correct DNA vector constructs. Selected bacterial colonies are picked with a sterile toothpick and dabbed into the PCR master mix or sterile water. The PCR is started with an extended time at 95˚C when standard polymerase is used or with a shortened denaturation step at 100˚C and special chimeric DNA polymerase.[17]
- Helicase-dependent amplification: This technique is similar to traditional PCR, but uses a constant temperature rather than cycling through denaturation and annealing/extension cycles. DNA Helicase, an enzyme that unwinds DNA, is used in place of thermal denaturation.[18]
- Hot-start PCR: This is a technique that reduces non-specific amplification during the initial set up stages of the PCR. The technique may be performed manually by heating the reaction components to the melting temperature (e.g., 95˚C) before adding the polymerase.[19] Specialized enzyme systems have been developed that inhibit the polymerase's activity at ambient temperature, either by the binding of an antibody[7] or by the presence of covalently bound inhibitors that only dissociate after a high-temperature activation step. Hot-start/cold-finish PCR is achieved with new hybrid polymerases that are inactive at ambient temperature and are instantly activated at elongation temperature.
- Intersequence-specific (ISSR) PCR: a PCR method for DNA fingerprinting that amplifies regions between some simple sequence repeats to produce a unique fingerprint of amplified fragment lengths.[20]
- Inverse PCR: a method used to allow PCR when only one internal sequence is known. This is especially useful in identifying flanking sequences to various genomic inserts. This involves a series of DNA digestions and self ligation, resulting in known sequences at either end of the unknown sequence.[21]
- Ligation-mediated PCR: This method uses small DNA linkers ligated to the DNA of interest and multiple primers annealing to the DNA linkers; it has been used for DNA sequencing, genome walking, and DNA footprinting. [22]
- Methylation-specific PCR (MSP): The MSP method was developed by Stephen Baylin and Jim Herman at the Johns Hopkins School of Medicine,[23] and is used to detect methylation of CpG islands in genomic DNA. DNA is first treated with sodium bisulfite, which converts unmethylated cytosine bases to uracil, which is recognized by PCR primers as thymine. Two PCR reactions are then carried out on the modified DNA, using primer sets identical except at any CpG islands within the primer sequences. At these points, one primer set recognizes DNA with cytosines to amplify methylated DNA, and one set recognizes DNA with uracil or thymine to amplify unmethylated DNA. MSP using qPCR can also be performed to obtain quantitative rather than qualitative information about methylation.
- Multiplex Ligation-dependent Probe Amplification (MLPA): permits multiple targets to be amplified with only a single primer pair, thus avoiding the resolution limitations of multiplex PCR (see below).
- Multiplex-PCR: The use of multiple, unique primer sets within a single PCR reaction to produce amplicons of varying sizes specific to different DNA sequences. By targeting multiple genes at once, additional information may be gained from a single test run that otherwise would require several times the reagents and more time to perform. Annealing temperatures for each of the primer sets must be optimized to work correctly within a single reaction, and amplicon sizes, i.e., their base pair length, should be different enough to form distinct bands when visualized by gel electrophoresis.
- Nested PCR: increases the specificity of DNA amplification, by reducing background due to non-specific amplification of DNA. Two sets of primers are being used in two successive PCR reactions. In the first reaction, one pair of primers is used to generate DNA products, which besides the intended target, may still consist of non-specifically amplified DNA fragments. The product(s) are then used in a second PCR reaction with a set of primers whose binding sites are completely or partially different from and located 3' of each of the primers used in the first reaction. Nested PCR is often more successful in specifically amplifying long DNA fragments than conventional PCR, but it requires more detailed knowledge of the target sequences.
- Overlap-extension PCR: is a genetic engineering technique allowing the construction of a DNA sequence with an alteration inserted beyond the limit of the longest practical primer length.
- Quantitative PCR (Q-PCR): is used to measure the quantity of a PCR product (preferably real-time). It is the method of choice to quantitatively measure starting amounts of DNA, cDNA or RNA. Q-PCR is commonly used to determine whether a DNA sequence is present in a sample and the number of its copies in the sample. The method with currently the highest level of accuracy is Quantitative real-time PCR. It is often confusingly known as RT-PCR (Real Time PCR) or RQ-PCR. QRT-PCR or RTQ-PCR are more appropriate contractions. RT-PCR commonly refers to reverse transcription PCR (see below), which is often used in conjunction with Q-PCR. QRT-PCR methods use fluorescent dyes, such as Sybr Green, or fluorophore-containing DNA probes, such as TaqMan, to measure the amount of amplified product in real time.
- RT-PCR: (Reverse Transcription PCR) is a method used to amplify, isolate or identify a known sequence from a cellular or tissue RNA. The PCR is preceded by a reaction using reverse transcriptase to convert RNA to cDNA. RT-PCR is widely used in expression profiling, to determine the expression of a gene or to identify the sequence of an RNA transcript, including transcription start and termination sites and, if the genomic DNA sequence of a gene is known, to map the location of exons and introns in the gene. The 5' end of a gene (corresponding to the transcription start site) is typically identified by a RT-PCR method, named RACE-PCR, short for Rapid Amplification of cDNA Ends.
- TAIL-PCR: Thermal asymmetric interlaced PCR is used to isolate unknown sequence flanking a known sequence. Within the known sequence TAIL-PCR uses a nested pair of primers with differing annealing temperatures; a degenerate primer is used to amplify in the other direction from the unknown sequence.[24]
- Touchdown PCR: a variant of PCR that aims to reduce nonspecific background by gradually lowering the annealing temperature as PCR cycling progresses. The annealing temperature at the initial cycles is usually a few degrees (3-5˚C) above the Tm of the primers used, while at the later cycles, it is a few degrees (3-5˚C) below the primer Tm. The higher temperatures give greater specificity for primer binding, and the lower temperatures permit more efficient amplification from the specific products formed during the initial cycles.[25]
- PAN-AC: This method uses isothermal conditions for amplification, and may be used in living cells.[26][27].
History
[засварлах | кодоор засварлах]A 1971 paper in the Journal of Molecular Biology by Kleppe and co-workers first described a method using an enzymatic assay to replicate a short DNA template with primers in vitro.[28] However, this early manifestation of the basic PCR principle did not receive much attention, and the invention of the polymerase chain reaction in 1983 is generally credited to Kary Mullis [29][30].
At the core of the PCR method is the use of a suitable DNA polymerase able to withstand the high temperatures of >90°C (>195°F) required for separation of the two DNA strands in the DNA double helix after each replication cycle. The DNA polymerases initially employed for in vitro experiments presaging PCR were unable to withstand these high temperatures. So the early procedures for DNA replication were very inefficient, time consuming, and required large amounts of DNA polymerase and continual handling throughout the process.
A 1976 discovery of Taq polymerase a DNA polymerase purified from the thermophilic bacterium, Thermus aquaticus, which naturally occurs in hot (50 to 80 °C (120 to 175 °F)) environments[8] paved the way for dramatic improvements of the PCR method. The DNA polymerase isolated from T. aquaticus is stable at high temperatures remaining active even after DNA denaturation,[9] thus obviating the need to add new DNA polymerase after each cycle. This allowed an automated thermocycler-based process for DNA amplification.
At the time he developed PCR in 1983, Mullis was working in Emeryville, California for Cetus Corporation, one of the first biotechnology companies. There, he was responsible for synthesizing short chains of DNA. Mullis has written that he conceived of PCR while cruising along the Pacific Coast Highway one night in his car.[29] He was playing in his mind with a new way of analyzing changes (mutations) in DNA when he realized that he had instead invented a method of amplifying any DNA region through repeated cycles of duplication driven by DNA polymerase. Mullis has credited the psychedelic drug LSD for his invention of the technique.[31]
In Scientific American, Mullis summarized the procedure: "Beginning with a single molecule of the genetic material DNA, the PCR can generate 100 billion similar molecules in an afternoon. The reaction is easy to execute. It requires no more than a test tube, a few simple reagents, and a source of heat."[32] He was awarded the Nobel Prize in Chemistry in 1993 for his invention,[2] seven years after he and his colleagues at Cetus first put his proposal to practice. However, some controversies have remained about the intellectual and practical contributions of other scientists to Mullis' work, and whether he had been the sole inventor of the PCR principle. (see main article: Kary Mullis)
Patent wars
[засварлах | кодоор засварлах]The PCR technique was patented by Cetus Corporation, where Mullis worked when he invented the technique in 1983. The Taq polymerase enzyme was also covered by patents. There have been several high-profile lawsuits related to the technique, including an unsuccessful lawsuit brought by DuPont. The pharmaceutical company Hoffmann-La Roche purchased the rights to the patents in 1992 and currently holds those that are still protected.
A related patent battle over the Taq polymerase enzyme is still ongoing in several jurisdictions around the world between Roche and Promega. The legal arguments have extended beyond the life of the original PCR and Taq polymerase patents, which expired on March 28, 2005.[33]
Ишлэл
[засварлах | кодоор засварлах]- ↑ Bartlett & Stirling (2003)—A Short History of the Polymerase Chain Reaction. In: Methods Mol Biol. 226:3-6
- ↑ 2.0 2.1 Karry Mullis Nobel Lecture, December 8, 1993
- ↑ Cheng S, Fockler C, Barnes WM, Higuchi R (1994). "Effective amplification of long targets from cloned inserts and human genomic DNA". Proc Natl Acad Sci. 91: 5695–5699. PMID 8202550.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 4.0 4.1 Joseph Sambrook and David W. Russel (2001). Molecular Cloning: A Laboratory Manual (3rd ed. ed.). Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press. ISBN 0-87969-576-5.
{{cite book}}:|edition=has extra text (help) Chapter 8: In vitro Amplification of DNA by the Polymerase Chain Reaction - ↑ Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI (2004). "Recent developments in the optimization of thermostable DNA polymerases for efficient applications". Trends Biotechnol. 22: 253–260. PMID 15109812.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Rychlik W, Spencer WJ, Rhoads RE (1990). "Optimization of the annealing temperature for DNA amplification in vitro". Nucl Acids Res. 18: 6409–6412. PMID 2243783.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 7.0 7.1 D.J. Sharkey, E.R. Scalice, K.G. Christy Jr., S.M. Atwood, and J.L. Daiss (1994). "Antibodies as Thermolabile Switches: High Temperature Triggering for the Polymerase Chain Reaction". Bio/Technology. 12: 506–509.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 8.0 8.1 Chien A, Edgar DB, Trela JM (1976). "Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus". J. Bacteriol. 174: 1550–1557. PMID 8432.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 9.0 9.1 Lawyer FC, Stoffel S, Saiki RK, Chang SY, Landre PA, Abramson RD, Gelfand DH (1993). "High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5' to 3' exonuclease activity". PCR Methods Appl. 2: 275–287. PMID 8324500.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ PCR from problematic templates. Focus 22:1 p.10 (2000).
- ↑ Helpful tips for PCR. Focus 22:1 p.12 (2000).
- ↑ "Chemical Synthesis, Sequencing, and Amplification of DNA (class notes on MBB/BIO 343)". Arizona State University. Татаж авсан: 29 October 2007.
- ↑ Saiki RK, Bugawan TL, Horn GT, Mullis KB, Erlich HA (1986). "Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes". Nature. 324: 163–166. PMID 3785382.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Stemmer WP, Crameri A, Ha KD, Brennan TM, Heyneker HL (1995). "Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides". Gene. 164: 49–53. PMID 7590320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Innis MA, Myambo KB, Gelfand DH, Brow MA. (1988). "DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA". Proc Natl Acad Sci USA. 85: 9436–4940. PMID 3200828.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Pierce KE and Wangh LJ (2007). "Linear-after-the-exponential polymerase chain reaction and allied technologies Real-time detection strategies for rapid, reliable diagnosis from single cells". Methods Mol Med. 132: 65–85. PMID 17876077.
- ↑ Pavlov AR, Pavlova NV, Kozyavkin SA, Slesarev AI (2006). "Thermostable DNA Polymerases for a Wide Spectrum of Applications: Comparison of a Robust Hybrid TopoTaq to other enzymes". In Kieleczawa J (ed.). DNA Sequencing II: Optimizing Preparation and Cleanup. Jones and Bartlett. pp. pp. 241-257. ISBN 0-7637338-3-0.
{{cite book}}:|pages=has extra text (help); Unknown parameter|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: authors list (link) - ↑ Myriam Vincent, Yan Xu and Huimin Kong (2004). "Helicase-dependent isothermal DNA amplification". EMBO reports. 5 (8): 795–800.
- ↑ Q. Chou, M. Russell, D.E. Birch, J. Raymond and W. Bloch (1992). "Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications". Nucleic Acids Research. 20: 1717–1723.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ E. Zietkiewicz, A. Rafalski, and D. Labuda (1994). "Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification". Genomics. 20 (2): 176–83.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Ochman H, Gerber AS, Hartl DL (1988). "Genetic applications of an inverse polymerase chain reaction". Genetics. 120: 621–623. PMID 2852134.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Mueller PR, Wold B (1988). "In vivo footprinting of a muscle specific enhancer by ligation mediated PCR". Science. 246: 780–786. PMID 2814500.
- ↑ Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996). "Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands". Proc Natl Acad Sci U S A. 93 (13): 9821–9826. PMID 8790415.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Y.G. Liu and R. F. Whittier (1995). "Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking". Genomics. 25 (3): 674–81.
- ↑ Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991). "'Touchdown' PCR to circumvent spurious priming during gene amplification". Nucl Acids Res. 19: 4008. PMID 1861999.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ David, F.Turlotte, E., (1998). "An Isothermal Amplification Method". C.R.Acad. Sci Paris, Life Science. 321 (1): 909–914.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ↑ Fabrice David (September–October 2002). "Utiliser les propriétés topologiques de l'ADN: une nouvelle arme contre les agents pathogènes" (PDF). Fusion.
{{cite web}}: CS1 maint: date format (link)(in French) - ↑ Kleppe K, Ohtsuka E, Kleppe R, Molineux I, Khorana HG (1971). "Studies on polynucleotides. XCVI. Repair replications of short synthetic DNA's as catalyzed by DNA polymerases". J. Mol. Biol. 56: 341–361.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ 29.0 29.1 Mullis, Kary (1998). Dancing Naked in the Mind Field. New York: Pantheon Books. ISBN 0-679-44255-3.
- ↑ Rabinow, Paul (1996). Making PCR: A Story of Biotechnology. Chicago: University of Chicago Press. ISBN 0-226-70146-8.
- ↑ Mullis, Kary (1998). Dancing Naked in the Mind Field. New York: Pantheon Books. p. 18. ISBN 0-679-44255-3.
{{cite book}}: More than one of|author=and|last=specified (help) - ↑ Mullis, Kary (1990). "The unusual origin of the polymerase chain reaction". Scientific American. 262 (4): 56–61, 64–5.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ Advice on How to Survive the Taq Wars ¶2: GEN Genetic Engineering News Biobusiness Channel: Article. May 1 2006 (Vol. 26, No. 9).
External links
[засварлах | кодоор засварлах]- PCR at Home - Amateur Scientist article in the July 2000 issue of Scientific American on performing PCR reactions with low-cost household materials.
- US Patent for PCR
- Narrated animation and step-through animation of PCR - From the educational multimedia company Sumanas. Adobe Flash required.
- Step-through animation of PCR - From Cold Spring Harbor's Dolan DNA Learning Center. Adobe Flash required.
- PCR exercise - A PCR problem will be generated and students will be requested to design a experiment to solve and simulate it.
