Хэрэглэгч:Tsogo3/Ноорог/Үндсэн өгүүллэгээс/Атом
| Атом | ||||||||
|---|---|---|---|---|---|---|---|---|
 | ||||||||
| Гелийн атомын бүтэц. Электрон үүлийн тархалтыг хар өнгөөр, түүний төвд ягаан өнгөөр цөмийг дүрслэв. Схемийг зургийн зүүн дээд хэсэгт байрлуулав (Нийлмэл цөмийн хувьд, протон ба нейтрон нь уг схем дээр үзүүлсэнтэй адил тэгш хэмтэйгээр байрлахгүй). | ||||||||
| Ангилал | ||||||||
| ||||||||
| Шинж чанар | ||||||||
|
[Хими]] ба физикийн шинжлэх ухаанд, атом (Грек ἄτομος буюу átomos нь хуваагдашгүй гэсэн утгатай) гэж химийн элементийг бүрдүүлэгч хамгийн жижиг хэсгийг хэлнэ.
Атом нь цөм \эерэг цэнэгтэй протон ба нейтраль цэнэгтэй протон\ , түүнийг хүрээлэн орших электрон үүлнээс \сөрөг цэнэгтэй электроноос тогтох\ тогтоно. Хэрэв атом нь электроныхоо тоотой ижил тооны нейтронтой бол цахилгаан цэнэгийн хувьд саармаг \нейтраль\ байна. Атом дахь протоны тоо нь химийн элементүүдийг тодорхойлох бөгөөд нейтроны тоо нь элементийн изотопийг тодорхойлоно.
Түүх
[засварлах | кодоор засварлах]The concept that matter is composed of discrete units and can not be divided into any arbitrarily tiny or small quantities has been around for thousands of years, but these ideas were founded in abstract, philosophical reasoning rather than experimentation and empirical observation. The nature of atoms in philosophy varied considerably over time and between cultures and schools, and often had spiritual elements. Nevertheless, the basic idea of the atom was adopted by scientists thousands of years later because it elegantly explained new discoveries in the field of chemistry.
The earliest references to the concept of atoms date back to ancient India in the 6th century BCE. [2] The Nyaya and Vaisheshika schools developed elaborate theories of how atoms combined into more complex objects (first in pairs, then trios of pairs). [3] The references to atoms in the West emerged a century later from Leucippus whose student, Democritus, systemized his views. In around 450 BCE, Democritus coined the term atomos, which meant "uncuttable". Though both the Indian and Greek concepts of the atom were based purely on philosophy, modern science has retained the name coined by Democritus.

In 1803, John Dalton used the concept of atoms to explain why elements always reacted in simple proportions, and why certain gases dissolved better in water than others. He proposed that each element consists of atoms of a single, unique type, and that these atoms could join to each other, to form chemical compounds.
In 1827 a British botanist Robert Brown used a microscope to look at dust grains floating in water. He called their erratic motion "Brownian motion". Albert Einstein would later demonstrate that this motion was due to the water molecules bombarding the grains.
In 1897, JJ Thomson, through his work on cathode rays, discovered the electron and its subatomic nature, which destroyed the concept of atoms as being indivisible units. Later, Thomson also discovered the existence of isotopes through his work on ionized gases.
Thomson believed that the electrons were distributed evenly throughout the atom, balanced by the presence of a uniform sea of positive charge. However, in 1909, the gold foil experiment was interpreted by Ernest Rutherford as suggesting that the positive charge of an atom and most of its mass was concentrated in a nucleus at the center of the atom (Rutherford model), with the electrons orbiting it like planets around a sun. In 1913, Niels Bohr added quantum mechanics into this model, which now stated that the electrons were locked or confined into clearly defined orbits, and could jump between these, but could not freely spiral inward or outward in intermediate states.
In 1926, Erwin Schrödinger, using Louis DeBroglie's 1924 proposal that all particles behave to an extent like waves, developed a mathematical model of the atom that described the electrons as three-dimensional waveforms, rather than point particles. A consequence of using waveforms to describe electrons is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at any point in time; this became known as the uncertainty principle. In this concept, for any given value of position one could only obtain a range of probable values for momentum, and vice versa. Although this model was difficult to visually conceptualize, it was able to explain many observations of atomic behavior that previous models could not, such as certain structural and spectral patterns of atoms bigger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described orbital zones around the nucleus where a given electron is most likely to exist.
In 1913, Frederick Soddy discovered that there appeared to be several elements at each position on the atomic table. The term isotope was coined by Margaret Todd as a suitable name for these elements. The development of the mass spectrometer allowed the exact mass of atoms to be measured. Francis William Aston used this technique to demonstrate that elements had isotopes of different mass. These isotopes varied by integer amounts, called the Whole Number Rule.[4] The explanation for these different atomic isotopes awaited the discovery of the neutron, a neutral-charged particle with a mass similar to the proton, by James Chadwick in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.
Бүтэц
[засварлах | кодоор засварлах]Атомын дотоод дахь бөөм
[засварлах | кодоор засварлах]Хэдийгээр атом гэдэг үг нь дахин хуваагдашгүй, хамгийн жижиг хэсэг гэсэн утгатай боловч, атом нь хэд хэдэн эгэл бөөмсүүдээс тогтоно. Үндсэн эгэл бөөмсүүд нь электрон, протон, нейтрон юм \ганцхан устөрөгч -1 нь нейтронгүй\.
The electron is by far the least massive of these particles at 9.11×10-31 kg, with a negative electrical charge and a size that is so small as to be currently unmeasurable.[5] Protons have a positive charge and a mass 1,836 times that of the electron, at 1.67×10-27 kg, although atomic binding energy changes can reduce this. Neutrons have no electrical charge and have a free mass of 1,839 times the mass of electrons.[6] Neutrons and protons have comparable dimensions—on the order of 2.5×10-15 m—although the 'surface' of these particles is not very sharply defined.[7]
Both protons and neutrons are themselves now thought to be composed of even more elementary particles, called quarks. The quark forms one of the two basic constituents of matter, the other being the lepton, of which the electron is an example. There are six different types of quarks, and each has a fractional electric charge of either +2/3 or −1/3. Protons are composed of two up quarks and one down quark, while a neutron consists of one up quark and two down quarks. The quarks are held together by the strong nuclear force, mediated by elementary particles called gluons.
Цөм
[засварлах | кодоор засварлах]Атомын бүх протон ба нейтроны холбоог нуклон гэж нэрлэх ба эдгээр нь хүнд, нягт атомын цөмийг үүсгэнэ.
Although the positive charge of protons causes them to repel each other, they are bound together with the neutrons by a short-ranged attractive potential called the residual strong force. The radius of a nucleus is approximately equal to fm, where A is total number of nucleons. This is much smaller than the radius of the atom, which is on the order of 105 fm.[8]
Atoms of the same element have the same number of protons, called the atomic number. Within a single element, the number of neutrons may vary, determining the isotope of that element. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay because of the weak force.
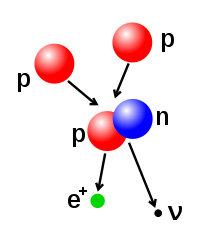
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when additional protons or neutrons collide with the nucleus. Nuclear fission is the opposite process, causing the nucleus to emit some amount of nucleons—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. In such processes which change the number of protons in a nucleus, the atom becomes an atom of a different chemical element.
The fusion of two nuclei that have lower atomic numbers than iron and nickel is an exothermic process that releases more energy than is required to bring them together. It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction. The net loss of energy from the fusion reaction also means that the mass of the fused nuclei is lower than the combined mass of the individual nuclei. The energy released (E) is described by Albert Einstein's mass–energy equivalence formula, E = mc², where m is the mass loss and c is the speed of light.
The mass of the nucleus is less than the sum of the masses of the separate particles. The difference between these two values is binding energy of the nucleus. It is the energy that is emitted when the individual particles come together to form the nucleus.[8] The binding energy per nucleon increases with increasing atomic number until iron or nickel is reached.[9] For heavier nuclei, the binding energy begins to decrease. That means fusion processes with nuclei that have higher atomic numbers is an endothermic process. These more massive nuclei can not undergo an energy-producing fusion reaction that can sustain the hydrostatic equilibrium of a star. Eventually, at sufficiently high atomic numbers, the binding energy becomes negative, resulting in an unstable nucleus.
Электрон үүл
[засварлах | кодоор засварлах]Электронууд нь цөмийг хүлээлэн байрлах ба электрон үүлийг үүсгэнэ. Электронууд нь цөм дэх протонуудтай цахилгаан соронзон хүчээр холбогдоно.
These electrons are bound to the protons in the nucleus by the electromagnetic force. The number of electrons associated with an atom is most easily changed, due to the lower energy of binding of electrons.
Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms which have either a deficit or a surplus of electrons are called ions. Electrons that are furthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.

Each electron in an atom exists at a particular energy state within a characteristic region about the nucleus that is defined by an atomic orbital. This mathematical function describes the wave-like behavior of the electron in a particular quantum state. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.
Шинж чанар
[засварлах | кодоор засварлах]Химийн элемент
[засварлах | кодоор засварлах]An element consists of all atoms that have the same number of protons in their nuclei. Each element can have multiple isotopes—nuclei with specific numbers of protons and neutrons. Even hydrogen, the simplest of elements, has isotopes deuterium and tritium. The known elements form a continual range of atomic numbers from hydrogen up to element 118, ununoctium. All known isotopes of elements with atomic numbers greater than 82 are radioactive.
The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties. Elements with similar chemical properties are aligned in vertical columns and are known as a group. The horizontal rows correspond to the filling of a quantum shell of electrons. Thus the elements at the far right have their outer shell completely filled with electrons, which results in chemically very inert elements known as the noble gases.
Elements that are missing just one electron needed to fill their outer shell are called halogens and form a column just to the left of the noble gases. Halogens display electronegativity because, during chemical reactions, they tend to acquire an extra electron to fill their outer shell. By contrast the alkali metals, along the left side of the table (with the exception of hydrogen), seek to lose the extra electron in the outer-most shell. These are highly reactive elements that readily react with halogens to form ionic salts.
Хэмжээ
[засварлах | кодоор засварлах]Атомын гадаад хүрээг яг тодорхойлох нь төвөгтэй учир, түүний хэмжээг ихэнхи тохиолдолд, хоёр буюу түүнээс дээш атомууд хоорондоо холбогдсон үед, тэдгээрийн хоорондын зайгаар тодорхойлдог байна. Атмын радиус нь үелэх систем дэх байрлалаас хамааран өөрчлөгдөнө. [10] Хамгийн жижиг атом болох гелийн атомын радиус 31 пм, хамгийн том нь болох цезийн атом 298 пм байна. Хэдийгээр устөрөгч нь гелигээс бага атомын дугаартай боловч, устөрөгчийн тооцоолсон радиус гелийнхээс 70% том байдаг.
Various analogies have been used to demonstrate the minuteness of the atom. A typical human hair is about 1 million carbon atoms in width. An HIV virion is the width of 800 carbon atoms and contains about 100 million atoms total. An E. coli bacterium contains perhaps 100 billion atoms, and a typical human cell roughly 100 trillion atoms. A speck of dust might contain 3 trillion atoms. A single drop of water contains about 2 sextillion (2×1021) atoms of oxygen, and twice as many hydrogen atoms.[11] If an apple was magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.[12]
Magnetic moment
[засварлах | кодоор засварлах]Elementary particles possess a quantum mechanical property known as spin. This property is equivalent to the possession of angular momentum, giving this property a directional component, although the particles themselves can not be said to be rotating. Electrons in particular are "spin-½" particles, as are protons and neutrons. The spin of an atom is determined by the spins of its constituent components, and how the spin is distributed and arranged among the sub-atomic components.
The spin of an atom determines its magnetic moment, and consequently the magnetic properties of each element. In many elements, the electrons are paired up with each other, with one of each pair of electrons in a spin up state and the other in the opposite, spin down state. Thus the spins cancel each other out, reducing the total magnetic dipole moment to zero. In ferromagnetic elements such as iron though, one of the electrons is unpaired and the atom can experience a net magnetic moment in the absense of an external magnetic field. When the magnetic moment of many ferromagnetic elements are lined up, the material can produce a measurable macroscopic field.
The nucleus of an atom can also have a net spin. Normally the alignment of these nuclei are aligned in random directions because of thermal equilibrium. However, for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.
Молекул
[засварлах | кодоор засварлах]
For gases and certain molecular liquids and solids (such as water and sugar), molecules are the smallest division of matter which retains chemical properties; however, there are also many solids and liquids which are made of atoms, but do not contain discrete molecules (such as salts, rocks, and liquid and solid metals). Thus, while molecules are common on Earth (making up all of the atmosphere and most of the oceans), most of the mass of the Earth (much of the crust, and all of the mantle and core) is not made of identifiable molecules, but rather represents atomic matter in other networked arrangements, all of which lack the particular type of small-scale interrupted order (i.e., small, strongly bound collections of atoms held to other collections of atoms by much weaker forces) that is associated with molecular matter.
Most molecules are made up of multiple atoms; for example, a molecule of water is a combination of two hydrogen atoms and one oxygen atom. The term 'molecule' in gases has been used as a synonym for the fundamental particles of the gas, whatever their structure. This definition results in a few types of gases (for example inert elements that do not form compounds, such as neon), which has 'molecules' consisting of only a single atom.[13]
Үүсэл
[засварлах | кодоор засварлах]Онолын хувьд, ертөнц дээрхи анхны цөмүүд \устөрөгч, гели, литийн ихэнхи, бараг бүх дейтри, гели-3-ийн атомын\ Их тэсрэлтийн дараагийн 3 минутанд явагдсан цөмийн синтезээр үүссэн байна. Анхны атом Их тэсрэлтээс хойш 380,000 жилийн дараа буюу ертөнц хангалттай хөрсний дараа электронууд цөмтэй нэгдэж эхэлснээр үүссэн байна.
Since then, atomic nuclei have been combined in stars through the process of nuclear fusion to generate atoms up to iron. Some atoms such as lithium-6 are generated in space through cosmic ray spallation. Elements heavier than iron were generated in supernovae through the r-process and in AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei. Some elements, such as lead, formed largely through the radioactive decay of heavier elements.
Most of the atoms that currently make up the Earth and all its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the solar system. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the earth through radiometric dating. Most of the helium on earth is a product of alpha-decay.
There are a few trace atoms on Earth that were not present at the beginning, nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere. Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions, including all the plutonium and technetium on the earth.
Мөн үзэх
[засварлах | кодоор засварлах]Ишлэл
[засварлах | кодоор засварлах]- Kenneth S. Krane, Introductory Nuclear Physics (1987)
- ↑ Matthew Champion, "Re: How many atoms make up the universe?", 1998
- ↑ Gangopadhyaya, Mrinalkanti. Indian Atomism: History and Sources. Atlantic Highlands, New Jersey: Humanities Press, 1981. ISBN 0-391-02177-X
- ↑ Teresi, Dick (2003). Lost Discoveries: The Ancient Roots of Modern Science. Simon & Schuster. pp. 213–214. ISBN 074324379X.
- ↑ Aston, Francis W. (1920). "The constitution of atmospheric neon". Philosophical Magazine. 39 (6): 449–455.
- ↑ Demtröder, Wolfgang (2002). Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics (1st Edition ed.). Springer. ISBN 3540206310.
{{cite book}}:|edition=has extra text (help) - ↑ Woan, Graham (2000). The Cambridge Handbook of Physics. Cambridge University Press. ISBN 0521575079.
- ↑ MacGregor, Malcolm H. (1992). The Enigmatic Electron. Oxford University Press. ISBN 0195218337.
- ↑ 8.0 8.1 Pfeffer, Jeremy I. (2000). Modern Physics: An Introductory Text. Imperial College Press. ISBN 1860942504.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ↑ Fewell, M. P. (1995). "The atomic nuclide with the highest mean binding energy". American Journal of Physics. 63 (7): 653–658. Татаж авсан: 2007-02-01.
- ↑ Dong, Judy (1998). "Diameter of an Atom". The Physics Factbook. Татаж авсан: 2007-11-19.
- ↑
Padilla, Michael J. (2002). Prentice Hall Science Explorer: Chemical Building Blocks. Upper Saddle River, New Jersey USA: Prentice-Hall, Inc. ISBN 0-13-054091-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Science textbook, Page 32: "There are 2,000,000,000,000,000,000,000 (that's 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen." - ↑ Richard Feynman (1995). Six Easy Pieces. The Penguin Group. ISBN 978-0-140-27666-4.
- ↑ Chandra, Sulekh. Comprehensive Inorganic Chemistry. New Age Publishers. ISBN 8122415121.
Гадаад холбоос
[засварлах | кодоор засварлах]- Atomic sizes
- How Atoms Work
- Wikibooks FHSST Physics Atom:The Atom
- Wikibooks Atomic structure
- Science aid - atomic structure A guide to the atom for teens.

![{\displaystyle {\begin{smallmatrix}1.2\cdot {\sqrt[{3}]{A}}\end{smallmatrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7d5a1afabf4f9ee32833a839bbe1faa70a18d828)