Хэрэглэгч:Bilguun.alt/Ноорог/Масс спектрометрчлэл
Масс спектрометрчлэл нь ионуудын масс-цэнэгийн харьцааг хэмждэг аналитик аргачлал юм.[1] Энэ аргачлалыг ихэвчлэн биет дээжний бүтэцийг тухайн дээжний найрлаганд буй бодисуудын массуудыг харуулсан масс спектрийг гаргах замаар тодорхойлоход ашиглагддаг. Масс спектрийг масс спектрометрээр хэмждэг.
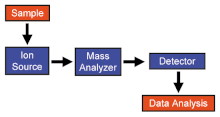
Бүх масс спектрометрүүд ионы үүсгүүр, масс анализатор, бүртгэгч систем гэсэн үндсэн гурван хэсгээс тогтдог. Масс спектрометр дэхь шатлалууд нь:
- Дээжнээс ион үүсгэх
- Өөр өөр масстай ионуудыг ялгах
- Тухайн масс бүхий ионуудын тоог бүртгэх
- Өгөгдөлийг цуглуулж масс спектрийг гаргах
Энэхүү аргачлалын хэрэглээнүүд нь:
- үл мэдэгдэх найрлагыг түүний найрлага дахь молекулууд эсвэл фракцуудын массаар нь тогтоох
- тухайн найрлаганд буй элементүүдийн изотопи бүтэцийг тодорхойлох
- тухайн найрлагын фракцыг ажиглах замаар найрлагын бүтэцийг тодорхойлох
- сайтар боловсруулсан аргаар найрлагын хэмжээг тогтоох (масс спектрометрчлэл нь шууд хэмжээг тогтоодоггүй)
- studying the fundamentals of gas phase ion chemistry (the chemistry of ions and neutrals in vacuum)
- determining other physical, chemical, or even biological properties of compounds with a variety of other approaches
Etymology
[засварлах | кодоор засварлах]The earliest devices that measured the mass-to-charge ratio of ions were called mass spectrographs because they were instruments that recorded a spectrum of mass values on a photographic plate.[2][3] Removing several letters, such as the bound morphemes and free morphemes, and combining the lingustical roots of spectr-um and phot-orgraph-ic plate creates the word spectrograph.[4] A mass spectroscope is similar to a mass spectrograph except that the beam of ions is directed onto a phosphor screen.[5] The suffix -scope here denotes the direct viewing of the spectra (range) of masses. A mass spectroscope configuration was used in early instruments when it was desired that the effects of adjustments be quickly observed. Once the instrument was properly adjusted, a photographic plate was inserted and exposed. The term mass spectroscope continued to be used even though the direct illumination of a phosphor screen was replaced by indirect measurements with an oscilloscope.[6] mass spectroscopy has been used in the past, and is a strictly correct etymology, literally an instrument for the viewing of a range of masses, but is now discouraged due to the possibility of confusion with light spectroscopy.[1] The terms mass spectroscopy and mass spectrometry are currently used, although the latter is strongly preferred.[7][1] Mass spectrometry is often abbreviated as mass-spec or simply as MS.[1]
History
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх History of mass spectrometry.
In 1886, Eugen Goldstein observed "rays" that travelled through the channels of a perforated cathode in a low pressure gas discharge and moved toward the anode, in the opposite direction to the negatively charged cathode rays. Goldstein called these positively charged anode rays "Kanalstrahlen" or canal rays. Wilhelm Wien found that strong electric or magnetic fields deflected the canal rays and, in 1899, constructed a device with parallel electric and magnetic fields that separated the positive rays according to their charge-to-mass ratio (e/m). Wien found that the charge-to-mass ratio depended on the nature of the gas in the discharge tube. English scientist J.J. Thomson later improved on the work of Wilhelm Wien by reducing the pressure to create a mass spectrograph. The processes that more directly gave rise to the modern version of the mass spectrometer were devised by Arthur Jeffrey Dempster and F.W. Aston in 1918 and 1919 respectively.
In 2002, the Nobel Prize in Chemistry was received by John Bennett Fenn for the development of electrospray ionization (ESI) and Koichi Tanaka for the development of soft laser desorption (SLD) in 1987. An improved SLD method, matrix-assisted laser desorption/ionization (MALDI), was developed in 1987 by Franz Hillenkamp and Michael Karas.[8]
Ажиллагааны хялбаршуулсан жишээ
[засварлах | кодоор засварлах]
Өөр өөр химийн бодисууд өөр өөр масстай байдаг баримт дээр тулгуурлан масс спектрометр нь дээжинд ямар ямар химийн бодис агуулагдаж буйг тодорхойлдог. Жишээлбэл масс спектрометрын эхний шатлалд хоолны давсыг (NaCl) уур болгож (хий болгон хувиргах) улмаар цахилгаан цэнэгтэй бөөмүүд (Na+ болон Cl-) болгон ионжуулж, ионууд болгон хувиргана. Натрийн ионууд нь 23 а.е.м масстай дан изотопи юм. Хлорын ионууд 35 а.е.м (~75%) болон 37 а.е.м (~25%) масс бүхий хоёр изотопууд юм. Эдгээр ионууд нь цэнэгтэй учир тэдгээрийн хурдны хэмжээ болон чиглэлийг нь цахилгаан эсвэл соронзон ороны тусламжтайгаар өөрчилж болно. Цахилгаан орон ионуудыг өндөр хурдтай болтол хурдасгах болно. Үүний дараа эдгээр ионуудыг соронзон оронд оруулах бөгөөд соронзон орон нь бөөмсийн хөдөлж буй чиглэл болон соронзон ороны шугамыг агуулсан хавтгайд перпендикуляр хүчээр ион бүрт үйлчлэх болно. Энэ хүчний үйлчлэрээ ионуудын хөдөлгөөн хазайх (шулуун шугамаар хөдлөхийн оронд муруй шугамаар хөдлөнө) бөгөөд хазайлтын зэрэг нь тэдгээрийн ионуудын масс-цэнэгийн (m/z) харьцаанаас шууд хамаарна. Хөнгөн ионууд нь хүнд ионуудыг бодвол илүү их хазайдаг. Үүний учирыг Ньютоны хоёр дугаар хуулиар тайлбарлаж болно. Бөөмийн хурдатгал нь түүний массаас урвуу хамааралтай. Тиймээс соронзон орон хөнгөн ионуудыг хүнд ионуудаас илүү их хазайлгана (траектор нь илүү бага радиустай тойрог болно гэсэн үг). Бүртгэгч улмаар ионын бөөм бүрийн хазайлтыг хэмжиж авдаг. Энэ хэмжилтийн үр дүнд үүсгүүрээс гарсан бүх ионуудын масс-цэнэгийн харьцааг гаргаж авах бөгөөд энэ мэдээлэл дээр үндэслэн жинхэнэ дээжний химийн найрлага (тухайлбал натри болон хлор хоюул дээжинд агуулагдаж буйг) болон бүрэлдүүлэгч хэсгүүдийн (тухайлбал 35Cl 37Cl хоёрын харьцаа өөрчлөгдсөн эсэх) изотопи найрлагыг нь тодорхойлдог.
Энэ нь сектор төхөөрөмжийн жишээ байв. Гэхдээ маш олон төрлийн масс спектрометрүүд байдаг. Тэдгээр масс спектрометрүүдийн нийтлэг зарчим нь бүгд ион гаргагч ионы үүсгүүртэй, гаргасан ионуудаа массаар нь ангилдаг анализатортай, мөн ялгаатай массуудын харьцангуй эрчимийг хэмждэг детектортай байдаг. Бүх масс спектрометрийн цаад зарчим нь цахилгаан болон соронзон орон дахь хийн фазын ионуудын зам (траектор) нь тэдгээрийн масс-цэнэгийн харьцаанаас хамаардагт оршдог бөгөөд энэ харьцааг ашиглан ионуудыг нэг нэгнээс нь ялгаж салгадаг.
Төхөөрөмж
[засварлах | кодоор засварлах]Ионы үүсгүүр
[засварлах | кодоор засварлах]Ионы үүсгүүр бол шинжилгээ хийх материалыг ионжуулдаг масс спектрометрийн бүрэлдхүүн хэсэг юм. Ионууд нь улмаар цахилгаан болон соронзон ороны тусламжтайгаар масс анализаторт хүргэгддэг.
Ионжуулалтын аргачлал нь масс спектрометрээр ямар төрлийн дээжийг шинжлэх боломжтойг тодорхойлдог. Цахилгаан ионжуулалт болон химийн ионжуулалтуудыг хий болон уурыг шинжлэхэд ашигладаг. Химийн ионжуулалтын үүсгүүрт шинжлэх гэж буй бодисыг химийн ион-молекулын урвалаар үүсгүүрт мөргөлдөөн үүсэх явцад ионжуулдаг. Шингэн болон хатуу биологийн дээжүүдийг шинжлэхэд хэрэглэдэг аргачлалуудад электро-шүршигч ионжуулалт (Жон Фений нээсэн) болон матрицын дэмжлэгтэй лазер ионжуулалтууд (МДЛИ, К. Танака мөн М. Карас болон Ф. Хилленкамп нарын нээсэн) ордог. Индүкцийн хосолмол плазм үүсгүүрийг олон янзын дээжнүүд дээр металын анализ хийхэд ихэвчлэн ашигладаг. Мөн glow discharge, field desorption (FD), fast atom bombardment (FAB), термошүршигч, desorption/ionization on silicon (DIOS), Direct Analysis in Real Time (DART), atmospheric pressure chemical ionization (APCI), secondary ion mass spectrometry (SIMS), spark ionization болон thermal ionisation гэсэн үүсгүүрүүд байдаг.[9] Ion Attachment Ionization is a newer soft ionization technique that allows for fragmentation free analysis.
Масс анализатор
[засварлах | кодоор засварлах]Масс анализатор ионуудыг масс-цэнэгийн харьцаанаас нь хамааруулан салгадаг. Бүх масс спектрометр нь вакуум дахь цахилгаан болон соронзон оронд буй цэнэгтэй бөөмсийн динамик дээр суурилдаг. Энд дараах хоёр хууль үйлчилдэг:
энд F нь ионд үйлчилж буй хүч, m нь ионы масс, a нь хурдатгал, q нь ионы цэнэг, E нь цахилгаан ороны хүчлэг бөгөөд v x B нь ионы хурд болон соронзон ороны вектор үржвэр юм.
Дээрх хоёр илэрхийлэлээс ионд үйлчилж буй хүчийг тооцолбол:
Энэхүү дифференциал тэгшитгэл нь цэнэгтэй бөөмсийн сонгодог тэгшитгэл юм. Бөөмийн анхны төлөвийн өгөгдлүүдийг мэдсэнээр бөөмийн хөдөлгөөнийг орон зай цаг хугацааны хувьд бүрэн тодорхойлно. Тиймээс энэ нь бүх масс спектрометрийн суурь юм. Мөн энэ нь ижил физик хэмжигдэхүүнтэй m/q бөөмс нь яг ижил хөдөлдөг. Эндээс харвал бүх масс спектрометр нь үнэндээ m/q-г хэмждэг бөгөөд нарийн ярьвал энэ төхөөрөмжийг масс-цэнэгийн спектрометр гэж нэрлэх ёстой. Өгөгдөлийг гаргахдаа энэ нь ерөнхийдөө хэмжээсгүй m/z ашигладаг (масс-цэнэгийн харьцаа гэдэг ч энэ нь массын тоо болон цэнэгийн тооны харьцаа юм) бөгөөд энд z нь ионы (z=q/e) эгэл цэнэгийн (e) тоо юм.
Статик эсвэл динамик орон ашигладаг, соронзон эсвэл цахилгаан орон ашигладаг олон төрлийн масс анализаторууд байдаг. Гэхдээ бүгд дээрхи хуулийн дагуу ажилладаг. Анализатор бүр өөрийн давуу тал болон сул талуудтай. Many mass spectrometers use two or more mass analyzers for tandem mass spectrometry (MS/MS). In addition to the more common mass analyzers listed below, there are other less common ones designed for special situations.
Сектор
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх sector instrument.
Сектор орон бүхий масс анализатор нь цахилгаан болон эсвэл соронзон оронг ашиглан цэнэгтэй бөөмсийн хурд болон траекторт нөлөөлдөг. Дээр үзүүлсэнчлэн сектор төхөөрөмжүүд нь масс анализатор дотор хурдассан ионуудын чиглэлийг өөрчилдөг. Соронзон болон цахилгаан оронд орсон ионуудын зам масс-цэнэгийн харьцаанаас хамааран муруйх бөгөөд их цэнэгтэй хурдан хөдөлж буй хөнгөн ионууд нь илүү их хазайдаг. Ионууд нь бүртгэгчид хүрэх үед тэдгээрийн харьцангуй зөрүүг хэмждэг.[10]
Time-of-flight
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх time-of-flight mass spectrometry.
Perhaps the easiest to understand is the time-of-flight (TOF) analyzer. It uses an electric field to accelerate the ions through the same potential, and then measures the time they take to reach the detector. If the particles all have the same charge, the kinetic energies will be identical, and their velocities will depend only on their masses. Lighter ions will reach the detector first.[11]
Quadrupole
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх Quadrupole mass analyzer.
Quadrupole mass analyzers use oscillating electrical fields to selectively stabilize or destabilize ions passing through a radio frequency (RF) quadrupole field. A quadrupole mass analyzer acts as a mass selective filter and is closely related to the Quadrupole ion trap, particularly the linear quadrupole ion trap except that it operates without trapping the ions and is for that reason referred to as a transmission quadrupole. A common variation of the quadrupole is the triple quadrupole.
Quadrupole ion trap
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх quadrupole ion trap.
The quadrupole ion trap works on the same physical principles as the QMS, but the ions are trapped and sequentially ejected. Ions are created and trapped in a mainly quadrupole RF potential and separated by m/q, non-destructively or destructively.
There are many mass/charge separation and isolation methods but most commonly used is the mass instability mode in which the RF potential is ramped so that the orbit of ions with a mass are stable while ions with mass become unstable and are ejected on the z-axis onto a detector.
Ions may also be ejected by the resonance excitation method, whereby a supplemental oscillatory excitation voltage is applied to the endcap electrodes, and the trapping voltage amplitude and/or excitation voltage frequency is varied to bring ions into a resonance condition in order of their mass/charge ratio.[12][13]
The cylindrical ion trap mass spectrometer is a derivative of the quadrupole ion trap mass spectrometer.
Linear quadrupole ion trap
[засварлах | кодоор засварлах]A linear quadrupole ion trap is similar to a QIT, but traps ions in a 2D quadrupole field, instead of a 3D quadrupole field as in a QIT. Thermo Fisher's LTQ ("linear trap quadrupole") is an example of the Linear ion trap.[14]
Fourier transform ion cyclotron resonance
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх Fourier transform mass spectrometry.
Fourier transform mass spectrometry, or more precisely Fourier transform ion cyclotron resonance MS, measures mass by detecting the image current produced by ions cyclotroning in the presence of a magnetic field. Instead of measuring the deflection of ions with a detector such as an electron multiplier, the ions are injected into a Penning trap (a static electric/magnetic ion trap) where they effectively form part of a circuit. Detectors at fixed positions in space measure the electrical signal of ions which pass near them over time producing cyclical signal. Since the frequency of an ion's cycling is determined by its mass to charge ratio, this can be deconvoluted by performing a Fourier transform on the signal. FTMS has the advantage of high sensitivity (since each ion is 'counted' more than once) and much high resolution and thus precision.[15][16]
Ion cyclotron resonance is an older mass analysis technique similar to FTMS except that ions are detected with a traditional detector. Ions trapped in a Penning trap are excited by an RF electric field until they impact the wall of the trap where the detector is located with ions of different mass being resolved in time.
Orbitrap
[засварлах | кодоор засварлах]- Энэ сэдэвийг илүү дэлгэрэнгүй үзэх Orbitrap.
The Orbitrap is the most recently introduced mass analyser. In the Orbitrap, ions are electrostatically trapped in an orbit around a central, spindle-shaped electrode. The electrode confines the ions so that they both orbit around the central electrode and oscillate back and forth along the central electrode's long axis. This oscillation generates an image current in the detector plates which is recorded by the instrument. The frequencies of these image currents depend on the mass to charge ratios of the ions in the Orbitrap. Mass spectra are obtained by Fourier transformation of the recorded image currents.
Similar to Fourier transform ion cyclotron resonance mass spectrometers, Orbitraps have a high mass accuracy, high sensitivity and a good dynamic range.[17]
Detector
[засварлах | кодоор засварлах]The final element of the mass spectrometer is the detector. The detector records the charge induced or current produced when an ion passes by or hits a surface. In a scanning instrument the signal produced in the detector during the course of the scan versus where the instrument is in the scan (at what m/q) will produce a mass spectrum, a record of ions as a function of m/q.
Typically, some type of electron multiplier is used, though other detectors including Faraday cups and ion-to-photon detectors are also used. Because the number of ions leaving the mass analyzer at a particular instant is typically quite small, significant amplification is often necessary to get a signal. Microchannel Plate Detectors are commonly used in modern commercial instruments.[18] In FTMS and Orbitraps, the detector consists of a pair of metal surfaces within the mass analyzer/ion trap region which the ions only pass near as they oscillate. No DC current is produced, only a weak AC image current is produced in a circuit between the electrodes. Other inductive detectors have also been used. [19]
Tandem mass spectrometry
[засварлах | кодоор засварлах]Tandem mass spectrometry (MS/MS) involves multiple steps of mass selection or analysis, usually separated by some form of fragmentation. A tandem mass spectrometer is one capable of multiple rounds of mass spectrometry. For example, one mass analyzer can isolate one peptide from many entering a mass spectrometer. A second mass analyzer then stabilizes the peptide ions while they collide with a gas, causing them to fragment by collision-induced dissociation (CID). A third mass analyzer then catalogs the fragments produced from the peptides. Tandem MS can also be done in a single mass analyzer over time as in a quadrupole ion trap. There are various methods for fragmenting molecules for tandem MS, including collision-induced dissociation (CID), electron capture dissociation (ECD), electron transfer dissociation (ETD), infrared multiphoton dissociation (IRMPD) and blackbody infrared radiative dissociation (BIRD). An important application using tandem mass spectrometry is in protein identification.[20]
Tandem mass spectrometry enables a variety of experiments. Although it allows for many uniquely designed experiments some types of experiments are commonly used and built into many commercial mass spectrometers. Examples of these include single reaction monitoring (SRM), multiple reaction monitoring (MRM) and precursor ion scan. In single reaction monitoring the first analyzer allows only a single mass through and the second analyzer monitors for a specifically defined fragment ion. MRM is nearly identical except the second analyzer monitors multiple user defined fragment ions. These monikers are most often used with scanning instruments where the second mass analysis event is duty cycle limited. These experiments are used to increase specificity of detection of known molecules such as in pharmacokinetic studies. Precursor ion scan refers to monitoring for a specific loss from the precursor ion. The first and second mass analyzers scan across the spectrum separated by a user defined m/z value. This experiment is used to detect specific motifs within unknown molecules.
Common Mass Spectrometer Configurations & Techniques
[засварлах | кодоор засварлах]When all of the elements (source, analyzer and detector) of a mass spectrometer are combined to form a complete instrument and the specific configuration becomes common a new name, often an abbreviation of one or more of the internal components, becomes attached to the specific configuration and can become, within certain circles, more well known than the specific internal components. The most ubiquitous example of this is MALDI-TOF, which simply refers to combining a Matrix-assisted laser desorption/ionization source with a Time-of-flight mass analyzer. The MALDI-TOF moniker is, however, often more widely recognized by the non-mass spectrometrist scientist than MALDI or TOF individually as if inseparable. Other examples include inductively coupled plasma-mass spectrometry (ICP-MS), accelerator mass spectrometry (AMS), Thermal ionization-mass spectrometry (TIMS) and spark source mass spectrometry (SSMS). Sometimes the use of the generic "MS" actually implies a very specific mass analyzer and detection system as with AMS, which is always sector based. In other cases there are common configurations that may be implied but not necessarily.
Certain applications of mass spectrometry have developed monikers that although technically referring to a broad application also tend to indicate a specific or a limited number of instrument configurations. An example of this is isotope ratio mass spectrometry (IRMS). Despite only specifically indicating an application, the use of a limited number of sector based mass analyzers is implied and the name is used to refer to both the application and the instrument used for the application.
Other Separation Techniques Combined with Mass spectrometry
[засварлах | кодоор засварлах]An important enhancement to the mass resolving and determining capacity of mass spectrometry is the combination of mass spectrometry with analysis techniques that resolve mixtures of compounds in a sample based on other characteristics before introduction into the mass spectrometer.
Gas chromatography
[засварлах | кодоор засварлах]- See also the main article on Gas chromatography-mass spectrometry
A common form of mass spectrometry is gas chromatography-mass spectrometry (GC/MS or GC-MS). In this technique, a gas chromatograph is used to separate different compounds. This stream of separated compounds is fed on-line into the ion source, a metallic filament to which voltage is applied. This filament emits electrons which ionize the compounds. The ions can then further fragment, yielding predictable patterns. Intact ions and fragments pass into the mass spectrometer's analyser and are eventually detected.[21]
Liquid chromatography
[засварлах | кодоор засварлах]- See also the main article on Liquid chromatography-mass spectrometry
Similar to gas chromatography MS (GC/MS), liquid chromatography mass spectrometry (LC/MS or LC-MS) separates compounds chromatographically before they are introduced to the ion source and mass spectrometer. It differs from GC/MS in that the mobile phase is liquid, usually a combination of water and organic solvents, instead of gas. Most commonly, an electrospray ionization source is used in LC/MS.
Ion mobility
[засварлах | кодоор засварлах]Ion mobility spectrometry/mass spectrometry (IMS/MS or IMMS) is a technique where ions are first separated by drift time through some pressure of neutral gas given an electrical potential gradient before being introduced into a mass spectrometer.[22]
The drift time is a measure of the radius relative to the charge of the ion. The duty cycle of IMS (time over which the experiment takes place) is longer than most mass spectrometers such that the mass spectrometer can sample along the course of the IMS separation. This produces data about the IMS separation and the mass-to-charge ratio of the ions in a manner similar to LC/MS.[23]
The duty cycle of IMS is short relative to liquid chromatography or gas chromatography separations and can thus be coupled to such techniques producing triply hyphenated techniques such as LC/IMS/MS.[24]
Data and analysis
[засварлах | кодоор засварлах]Data representations
[засварлах | кодоор засварлах]Mass spectrometry produces various types of data. The most ubiquitous data representation is the mass spectrum.
Certain types of mass spectrometry data are best represented as a mass chromatogram. Types of chromatograms include selected ion monitoring (SIM), total ion current (TIC), and selected reaction monitoring chromatogram (SRM), among many others.
Other types of mass spectrometry data are well represented as a contour map. In this form, the mass-to-charge is on one axis, intensity on recorded on another axis, and an additional experimental parameter, such as time, is recorded on the third axis. This results in a three dimensional representation of the data.
Data analysis
[засварлах | кодоор засварлах]Basics
Mass spectrometry data analysis is a complicated subject matter that is very specific to the type of experiment producing the data. There are several general subdivisions of data that are fundamental to beginning to understand any data.
Many mass spectrometers work in either negative ion mode or positive ion mode. It is very important to know whether the observed ions are negatively or positively charged. This is often important in determining the neutral mass but it also indicates something about the nature of the molecules.
There are many different types of ion sources that behave very differently from each other. A source such as an electron ionization source produces many fragments and mostly odd electron species with one charge, whereas a source such as an electrospray source usually produces quasimolecular even electron species that may be multiply charged.
Tandem mass spectrometry purposely produces fragment ions post-source and can drastically change the sort of data achieved by an experiment.
By understanding the origin of a sample certain expectations can be assumed. For example, if the sample is coming from a synthesis/manufacturing process impurities are likely to be present that are related to the major component. If the sample is a relatively crude preparation of a biological sample, the sample likely contains a certain amount of salt that may form adducts with the analyte molecules in certain analyses.
Results can also depend heavily on how the sample was prepared and how it was run/introduced. An important example is which matrix was used for MALDI spotting, since much of the energetics of the desorption/ionization event is controlled by the matrix rather than the laser power. Sometimes samples are spiked with sodium or another ion-carrying species to produce adducts rather than a protonated species.
The most commonly overlooked basic question by non-mass spectrometrists trying to use mass spectrometry or interact with a mass spectrometrist is what is the over-arching goal of the project. To interpret data one must know the desired outcome (and have collected the right data in the first place). There are many bits of information that can be gleaned from mass spectrometry data, such as the masses of the molecules, the purity of the sample, and the structure of the molecules. Each of these questions requires a different approach. Simply asking for a "mass-spec" will most likely not answer the real question at hand.
Applications
[засварлах | кодоор засварлах]Isotope ratio MS: isotope dating and tracking
[засварлах | кодоор засварлах]
Mass spectrometry is also used to determine the isotopic composition of elements within a sample. Differences in mass among isotopes of an element are very small, and the less abundant isotopes of an element are typically very rare, so a very sensitive instrument is required. These instruments, sometimes referred to as isotope ratio mass spectrometers (IR-MS), usually use a single magnet to bend a beam of ionized particles towards a series of Faraday cups which convert particle impacts to electric current. A fast on-line analysis of deuterium content of water can be done using Flowing afterglow mass spectrometry, FA-MS. Probably the most sensitive and accurate mass spectrometer for this purpose is the accelerator mass spectrometer (AMS). Isotope ratios are important markers of a variety of processes. Some isotope ratios are used to determine the age of materials for example as in carbon dating. Labelling with stable isotopes is also used for protein quantification. (see Protein quantitation below)
Trace gas analysis
[засварлах | кодоор засварлах]Several techniques use ions created in a dedicated ion source injected into a flow tube or a drift tube: selected ion flow tube (SIFT-MS), and proton transfer reaction (PTR-MS), are variants of chemical ionization dedicated for trace gas analysis of air, breath or liquid headspace using well defined reaction time allowing calculations of analyte concentrations from the known reaction kinetics without the need for internal standard or calibration.
Atom Probe
[засварлах | кодоор засварлах]An atom probe is an instrument that combines time-of-flight mass spectrometry and field ion microscopy (FIM) to map the location of individual atoms.
Pharmacokinetics
[засварлах | кодоор засварлах]Pharmacokinetics is often studied using mass spectrometry because of the complex nature of the matrix (often blood or urine) and the need for high sensitivity to observe low dose and long time point data. The most common instrumentation used in this application is LC-MS with a triple quadrupole mass spectrometer. Tandem mass spectrometry is usually employed for added specificity. Standard curves and internal standards are used for quantitation of usually a single pharmaceutical in the samples. The samples represent different time points as a pharmaceutical is administered and then metabolized or cleared from the body. Blank or t=0 samples taken before administration are important in determining background and insuring data integrity with such complex sample matrices. Much attention is paid to the linearity of the standard curve; however it is not uncommon to use curve fitting with more complex functions such as quadratics since the response of most mass spectrometers is less than linear across large concentration ranges.[25][26][27]
There is currently considerable interest in the use of very high sensitivity mass spectrometry for microdosing studies, which are seen as a promising alternative to animal experimentation.
Protein characterization
[засварлах | кодоор засварлах]Mass spectrometry is an important emerging method for the characterization of proteins. The two primary methods for ionization of whole proteins are electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). In keeping with the performance and mass range of available mass spectrometers, two approaches are used for characterizing proteins. In the first, intact proteins are ionized by either of the two techniques described above, and then introduced to a mass analyser. This approach is referred to as "top-down" strategy of protein analysis. In the second, proteins are enzymatically digested into smaller peptides using an agent such as trypsin or pepsin. Other proteolytic agents are also used. The collection of peptide products are then introduced to the mass analyser. This peptide mass fingerprinting (PMF) approach of protein analysis is also referred to as the "bottom-up" approach.
Space exploration
[засварлах | кодоор засварлах]As a standard method for analysis several mass spectrometers have reached other planets and moons. Two were taken to Mars by the Viking program. In early 2005 the Cassini-Huygens mission delivered a specialized GC-MS instrument aboard the Huygens probe through the atmosphere of Titan, the largest moon of the planet Saturn. This instrument analyzed atmospheric samples along its descent trajectory and was able to vaporize and analyze samples of Titan's frozen, hydrocarbon covered surface once the probe had landed. These measurements compare the abundance of isotope(s) of each particle comparatively to earth's natural abundance.[28]
Mass spectrometers are also widely used in space missions to measure the composition of plasmas. For example, the Cassini spacecraft carries the Cassini Plasma Spectrometer (CAPS),[29] which measures the mass of ions in Saturn's magnetosphere.
Respired Gas Monitor
[засварлах | кодоор засварлах]Mass spectrometers were used in hospitals for respiratory gas analysis beginning around 1975 through the end of the century, some are likely still in use but none are currently being manufactured.[30]
Found mostly in the operating room they were a part of a complex system in which respired gas samples from patients undergoing anesthesia were drawn into the instrument through a valve mechanism designed to sequentially connect up to 32 rooms to the mass spectrometer. A computer directed all operations of the system, the data collected from the mass spectrometer was delivered to the individual rooms for the anesthesiologist to use.
This magnetic sector mass spectrometer's uniqueness may have been the fact that a plane of detectors, each purposely positioned to collect all of the ion species expected to be in the samples, allowed the instrument to simultaneously report all of the patient respired gases. Although the mass range was limited to slightly over 120 u, fragmentation of some of the heavier molecules negated the need for a higher detection limit.[31]
See also
[засварлах | кодоор засварлах]- Mass spectrometry software
- Electron spectrometer
- Atom probe
- Calutron
- Helium mass spectrometer
- Ion attachment mass spectrometry
- MALDI imaging
- Membrane introduction mass spectrometry
- Secondary ionisation
- Taylor cone
Manufacturers
[засварлах | кодоор засварлах]- Agilent Technologies
- Bruker Daltonics
- CovalX
- JEOL
- LECO Corporation
- MDS SCIEX / Applied Biosystems
- PerkinElmer
- Shimadzu Corporation
- Thermo Fisher Scientific
- Waters Corporation
- Varian
References
[засварлах | кодоор засварлах]- ↑ 1.0 1.1 1.2 1.3 Sparkman, O. David (2000). Mass spectrometry desk reference. Pittsburgh: Global View Pub. ISBN 0-9660813-2-3.
- ↑ Squires, Gordon (1998). "Francis Aston and the mass spectrograph". Dalton Transactions: 3893–3900. doi:10.1039/a804629h. Татаж авсан: 2007-12-06.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ KM Downard (2007). "Francis William Aston - the man behind the mass spectrograph". European Journal of Mass Spectrometry. 13 (3): 177–190. doi:10.1255/ejms.878.
- ↑ Harper, Douglas. "Spectrum." Online Etymology Dictionary. Nov. 2001. Accessed 07-12-2007. Note: This part of the article only makes descriptive claims about the information found in the primary source, the accuracy and applicability of which is easily verifiable by any reasonable, educated person without specialist knowledge. (See WP:PSTS)
- ↑ J.J., Thomson (1913). Rays Of Positive Electricity and Their Application to Chemical Analysis. London: Longman's Green and Company.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ Siri, William (August 1947). "Mass spectroscope for analysis in the low-mass range". Review of Scientific Instruments. 18 (8): 540–545. doi:10.1063/1.1740998. Татаж авсан: 2007-12-06.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ Price, Phil (1991). "Standard definitions of terms relating to mass spectrometry. A report from the Committee on Measurements and Standards of the American Society for Mass Spectrometry". Journal of the American Society for Mass Spectrometry. 2 (4): 336–348. doi:10.1016/1044-0305(91)80025-3 . Татаж авсан: 2007-12-06.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help) - ↑ Measuring Mass: From Positive Rays to Proteins by Michael A. Grayson (Editor) (ISBN 0-941901-31-9)
- ↑ A. P. Bruins (1991). "Mass spectrometry with ion sources operating at atmospheric pressure". Mass Spectrometry Reviews. 10 (1): 53–77. doi:10.1002/mas.1280100104.
- ↑ Extending the mass range of a sector mass spectrometer, John S Cottrell, Roger J Greathead, Mass Spectrometry Reviews Vol 5, 1986. pp 215-247
- ↑ Time-of-flight mass analyzers H. Wollnik, Mass Spectrometry Reviews, Vol 12, 1993, pp 89-114
- ↑ Paul W., Steinwedel H. (1953). "Ein neues Massenspektrometer ohne Magnetfeld". RZeitschrift für Naturforschung A 8 (7): 448-450
- ↑ R. E. March (2000). "Quadrupole ion trap mass spectrometry: a view at the turn of the century". International Journal of Mass Spectrometry. 200 (1–3): 285–312. doi:10.1016/S1387-3806(00)00345-6.
- ↑ Schwartz, Jae C. (June 2002). "A two-dimensional quadrupole ion trap mass spectrometer". Journal of the American Society for Mass Spectrometry. Elsevier Science B.V. 13 (6): 659–669. doi:10.1016/S1044-0305(02)00384-7.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ M. B. Comisarow and A. G. Marshall (1974). "Fourier transform ion cyclotron resonance spectroscopy". Chemical Physics Letters. 25 (2): 282–283. doi:10.1016/0009-2614(74)89137-2.
- ↑ Marshall, A. G.; Hendrickson, C. L.; Jackson, G. S. (1998). "Fourier transform ion cyclotron resonance mass spectrometry: a primer". Mass Spectrometry Reviews. 17 (1): 1–34. doi:10.1002/(SICI)1098-2787(1998)17:1%3C1::AID-MAS1%3E3.0.CO;2-K.
{{cite journal}}: Unknown parameter|doilabel=ignored (help)CS1 maint: multiple names: authors list (link) - ↑ Q. Hu, R. J. Noll, H. Li, A. Makarov, M. Hardman and R. G. Cooks (2005). "The Orbitrap: a new mass spectrometer". Journal of Mass Spectrometry. 40 (4): 430–443. doi:10.1002/jms.856.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ F. Dubois, R. Knochenmuss, R. Zenobi, A. Brunelle, C. Deprun and Y. L. Beyec (1999). "A comparison between ion-to-photon and microchannel plate detectors". Rapid Communications in Mass Spectrometry. 13 (9): 786–791. doi:10.1002/(SICI)1097-0231(19990515)13:9%3C786::AID-RCM566%3E3.0.CO;2-3.
{{cite journal}}: Unknown parameter|doilabel=ignored (help)CS1 maint: multiple names: authors list (link) - ↑ M. A. Park, J. H. Callahan and A. Vertes (1994). "An inductive detector for time-of-flight mass spectrometry". Rapid Communications in Mass Spectrometry. 8 (4): 317–322. doi:10.1002/rcm.1290080407.
- ↑ Robert K. Boyd (1994). "Linked-scan techniques for MS/MS using tandem-in-space instruments". Mass Spectrometry Reviews. 13 (5–6): 359–410. doi:10.1002/mas.1280130502.
- ↑ Eiceman, G.A. (2000). Gas Chromatography. In R.A. Meyers (Ed.), Encyclopedia of Analytical Chemistry: Applications, Theory, and Instrumentation, pp. 10627. Chichester: Wiley. ISBN 0-471-97670-9
- ↑ Verbeck, GF and Ruotolo, BT and Sawyer, HA and Gillig, KJ and Russell, DH (2002). "A fundamental introduction to ion mobility mass spectrometry applied to the analysis of biomolecules}". J Biomol Tech (http://jbt.highwire.org/cgi/content/abstract/13/2/56). 13 (2): 56–61.
{{cite journal}}:|access-date=requires|url=(help);|format=requires|url=(help); Cite has empty unknown parameter:|coauthors=(help); External link in|format= - ↑ L. M. Matz, G. R. Asbury and H. H. Hill (2002). "Two-dimensional separations with electrospray ionization ambient pressure high-resolution ion mobility spectrometry/quadrupole mass spectrometry". Rapid Communications in Mass Spectrometry. 16 (7): 670–675. doi:10.1002/rcm.623.
- ↑ Rena A. Sowell, Stormy L. Koeniger, Stephen J. Valentine, Myeong Hee Moon and David E. Clemmer (2004). "Nanoflow LC/IMS-MS and LC/IMS-CID/MS of Protein Mixtures". Journal of the American Society for Mass Spectrometry. 15 (9): 1341–1353. doi:10.1016/j.jasms.2004.06.014.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Increasing Speed and Throughput When Using HPLC-MS/MS Systems for Drug Metabolism and Pharmacokinetic Screening, Y. Hsieh and W.A. Korfmacher, Current Drug Metabolism Volume 7, Number 5, 2006, Pp. 479-489
- ↑ Covey TR, Lee ED, Henion JD. 1986. High-speed liquid chromatography/tandem mass spectrometry for the determination of drugs in biological samples. Anal Chem 58:2453-2460.
- ↑ Thermospray liquid chromatography/mass spectrometry determination of drugs and their metabolites in biological fluids. Covey TR et al. Anal Chem. 1985 Feb;57(2):474-81
- ↑ S. Petrie and D. K. Bohme (2007). "Ions in space". Mass Spectrometry Reviews. 26 (2): 258–280. doi:10.1002/mas.20114.
- ↑ http://caps.space.swri.edu/
- ↑ Expired gas monitoring by mass spectrometry in a respiratory intensive care unit. Riker JB, Haberman B. Crit Care Med. 1976 Sep-Oct;4(5):223-9
- ↑ J. W. W. Gothard, C. M. Busst, M. A. Branthwaite, N. J. H. Davies and D. M. Denison (1980). "Applications of respiratory mass spectrometry to intensive care". Anaesthesia. 35 (9): 890–895. doi:10.1111/j.1365-2044.1980.tb03950.x.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Bibliography
[засварлах | кодоор засварлах]- Tureček, František; McLafferty, Fred W. (1993). Interpretation of mass spectra. Sausalito, Calif: University Science Books. ISBN 0-935702-25-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Edmond de Hoffman (2001). Mass Spectrometry: Principles and Applications (2nd ed. ed.). John Wiley and Sons. ISBN 0-471-48566-7.
{{cite book}}:|edition=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Downard, Kevin (2004). Mass Spectrometry - A Foundation Course. Cambridge UK: Royal Society of Chemistry. ISBN 0-85404-609-6.
{{cite book}}: Check|isbn=value: checksum (help) - Siuzdak, Gary (1996). Mass spectrometry for biotechnology. Boston: Academic Press. ISBN 0-12-647471-0.
- Dass, Chhabil (2001). Principles and practice of biological mass spectrometry. New York: John Wiley. ISBN 0-471-33053-1.
- Jnrgen H. Gross. Mass Spectrometry: A Textbook. Berlin: Springer-Verlag. ISBN 3-540-40739-1.
- Muzikar, P., et al., "Accelerator Mass Spectrometry in Geologic Research", Geological Society of America Bulletin v. 115 (2003) p. 643 - 654.
- David O. Sparkman. Mass Spectrometry Desk Reference. Pittsburgh: Global View Pub. ISBN 0-9660813-9-0.
- Tuniz, C. (1998). Accelerator mass spectrometry: ultrasensitive analysis for global science. Boca Raton: CRC Press. ISBN 0-8493-4538-3.
External links
[засварлах | кодоор засварлах]| Wikibooks: Bilguun.alt/Ноорог/Масс спектрометрчлэл – Сурах бичиг, зааврууд |
| Wiktionary: Bilguun.alt/Ноорог/Масс спектрометрчлэл – Энэ үгийг тайлбар толиос харна уу |
- Science Aid: Mass Spectrometry Easy to understand resource for high school level
- Overview of resources on Mass Spectrometry
- A History of Mass Spectrometry (Scripps)
- Mass spectrometer simulation An interactive application simulating the console of a mass spectrometer
- Nature Protocols An on-line resource containing protein - mass spectrometry methods
- Professional organizations







